Isobutane vs Butane – Butane vs Propane vs LPG Gas – Properties
 What is the real difference between butane vs propane vs LPG gas and isobutane vs butane?
What is the real difference between butane vs propane vs LPG gas and isobutane vs butane?
Propane, butane and isobutane gas are all consider to be LPG – Liquefied Petroleum Gas.
Considering isobutane vs butane, butane (C4H10) and isobutane (C4H10) have the same chemical formula, as isobutane is isomer of butane.
Propane (C3H8) is used primarily as fuel whilst butane and isobutane gas are used as propellant gases.
Propane, butane and isobutane also have some different physical properties including propane boiling point, butane boiling point, and pressure.
First, some short summary answers:
Isobutane or i-Butane
 Isobutane (i-butane) is an isomer of butane.
Isobutane (i-butane) is an isomer of butane.
As an isomer, the chemical formula of isobutane (C4H10) is the same as butane but has a different molecular structure.
A process called isomerization is used to convert butane (n-butane) to isobutane (i-butane).
Other Names for Isobutane: i-Butane, i Butane or Methylpropane
i-Butane or i Butane are just other names for isobutane.
Methylpropane is yet another name for isobutane.
Isobutane Gas – Isobutane vs Butane – Isobutane Refrigerant – Isobutane Canister
As with normal butane gas, isobutane gas is a flammable hydrocarbon gas that is liquefied through pressurisation.
So, isobutane has the same butane chemical formula — C4H10 — but isobutane has a different arrangement of its atoms, as you can see in the 3-D isobutane model images. (Isobutane molecule model shown)
Isobutane gas also has different physical properties from normal butane (n-butane).
 In addition to being used as a fuel, isobutane refrigerant (R600a isobutane refrigerant) is commonly used as is isobutane gas propellant, sold as R600a in an isobutane canister.
In addition to being used as a fuel, isobutane refrigerant (R600a isobutane refrigerant) is commonly used as is isobutane gas propellant, sold as R600a in an isobutane canister.
Whilst isobutane (isobutane gas) is flammable, there have been few problems in the millions of R600a isobutane refrigerant units worldwide.
R600a isobutane refrigerant has very low global warming potential and insignificant ozone depletion potential.
However, isobutane main use is in refineries to increase octane of gasoline and make it cleaner burning.
Isobutane gas is classified as LPG, along with propane gas, butane gas and mixes of these gases.
Considering isobutane vs butane, as previously explained, isobutane (i-butane) is an isomer of butane (n-butane).
Chemical formula of isobutane (C4H10) is the same as butane but the difference between butane and isobutane is a different molecular structure.
Isobutane gas is converted from butane in a process called isomerization.
Isobutane Uses
Isobutane uses are primarily in refineries, as a gasoline – petrol – additive.
There, isobutane is processed through an alkylation unit to make an alkylate.
Isobutane is used to make isooctane, a high octane gasoline component, which increases the octane rating and anti-knock properties of gasoline.
Isobutane (isobutane gas) is rated at 100 points on the octane rating scale.
As previously mention, isobutane (isobutane gas) is frequently used as an isobutane refrigerant, called r600a isobutane refrigerant distributed in an isobutane canister.
Another important use of isobutane is as a feed stock for plastics.
It is used to manufacture propylene oxide for use in making polyurethane plastics.
Isobutane is also used as a solvent.
Is Isobutane Toxic – Isobutane Toxicity
Isobutane toxicity is mostly a case of displacing oxygen, as an asphyxiant, at high enough concentrations.
Liquid isobutane can also cause frostbite if it makes contact with bare skin.
Isobutane side effects in high concentrations can cause dizziness, lightheadedness, headache, nausea, and even unconsciousness.
In very high concentrations isobutane can displace oxygen in the air, leading to rapid suffocation.
You should consult an Isobutane SDS for full information on isobutane toxicity.
Isobutane vs Butane – Difference Between Butane and Isobutane?
Comparing isobutane vs butane, there isn’t much difference between butane (n-butane) and isobutane (i-butane).
Both butane and isobutane are classified as LPG.
As previously mentioned, isobutane even has the same chemical formula as butane — C4H10 — just with a different arrangement of isobutane atoms.
There are two noticeable differences between isobutane vs butane:
1. The n butane boiling point temperature is about 11°C higher than isobutane boiling point.
2. Pressure is probably the biggest difference between butane and isobutane, with isobutane gas at 310.9 kPa and n butane gas at 215.1 kPa (both at 21ºC).
Is LPG Gas the Same as Propane – Is Propane the Same as LPG – LPG or Propane – Is LPG Propane
Is propane the same as LPG? Is LPG gas the same as propane? Is LPG propane? Is propane and LP gas the same?
Is it LPG or propane?
Propane is LPG but not all LPG is propane.
LPG – liquefied petroleum gas – can also contain butane, isobutane, ethane and pentane, flammable hydrocarbon gases liquefied under pressure.
Is LP Gas the Same as Propane – Is Propane and LP Gas the Same?
Is LP Gas the Same as Propane – Is Propane and LP Gas the Same?
No, LP gas is not the same as propane. Neither are propane and LP gas the same.
As above, propane is LP gas but not all LP gas is propane.
Butane vs Propane – How do Butane and Propane Gas Differ? Butane Boiling Point
 When comparing butane vs propane, the most important differences are propane and butane boiling point and vapour pressure.
When comparing butane vs propane, the most important differences are propane and butane boiling point and vapour pressure.
Propane boiling point is lower, at -42°C vs -0.4°C for butane boiling point.
So, propane will continue to vaporise – turn to gas – even in colder climates, down to -42°C vs butane boiling point at a higher -0.4°C.
Butane gas has a lower vapour pressure at a given temperature, being about ¼ that of propane gas.
This lower pressure of butane gas is advantageous for some propellant applications.
Propane vs Butane Energy Content
Propane vs butane energy content is also different.
Butane has a higher energy content by volume, as it has a higher density. Relative density compared to air is 2.00 for butane vs 1.53 for propane.
However, propane energy content is slightly higher by weight but lower by volume.
This seeming inconsistency of butane vs propane gas is as a result of butane and propane liquid gases having a different specific gravity.
Propane vs Butane Applications
Propane is more widely used with applications ranging from home heating, hot water and cooking to heavy industrial applications like boilers, dryers and kilns.
Propane is also used in cold weather applications.
Butane is often used for small portable heating appliances, including camping stoves.
The lower vapour pressure provided by butane makes it popular as a propellant gas.
Propane vs Butane Performance
The fact that the propane boiling point is lower, at -42°C vs -0.4°C than for butane boiling point means it performs better in colder climates, as the gas bottle contents vaporise or continue to stay gaseous at the lower temperatures.
Propane vs Butane Storage
Both propane and butane are stored in similar refillable gas bottles when used for none portable applications.
For portable applications, like camping, butane is available in single use, non-refillable containers.
The lower vapour pressure of butane can also allow for lighter weight cylinders, as they do not need to be as pressure resistant.
Butane Gas Bottle vs Propane Gas Bottle
You would be hard pressed to tell the difference between a butane gas bottle and a propane gas bottle, unless it gets cold.
If it gets down to -0.4°C, butane boiling point, then the butane gas bottle will stop working whilst the propane gas bottle will still be fine.
As explained above, this is because butane boiling point is higher, so a butane gas bottle will stop vaporizing at or below -0.4°C.
What is Butane (n-butane)? Butane Gas Bottle
Butane (n-butane) is a flammable hydrocarbon gas that is liquefied through pressurisation and stored in a butane gas bottle or tank.
Butane comes from natural gas processing and oil refining.
Butane is commonly used as a fuel, propellant and refrigerant, as well as a petrochemical feedstock.
The butane chemical formula (butane molecular formula) is C4H10. (Butane molecule model shown)
Butane (n-butane) also falls under the category of an LPG gas.
Butane is classified as LPG, along with propane, isobutane and mixtures of these gases.
Butane gas is supplied to businesses that require Butane, as opposed to propane.
Butane gas has some specific applications where it has advantages over propane.
n-Butane or n Butane
Both n-Butane or n Butane are just other names for regular butane gas.
What is Propane? Is Propane Gas LPG? Is LPG Propane? Is LP Gas Propane?
Propane is a flammable hydrocarbon gas that is liquefied through pressurisation.
Propane gas comes from natural gas processing and oil refining.
It is commonly used for heating and cooking.
Is propane gas LPG? Yes, propane gas is classified as an LPG gas.
Is LPG propane (Is LP gas propane)? Is propane and LP gas the same? Sometimes, but not always. LPG can also be butane or isobutane.
So, propane is LPG but not all LPG is propane.
LPG or propane is saying the same thing in Australia, as LPG is propane in Australia but not necessarily in other countries..
Propane is the gas that is supplied to virtually all homes and most businesses that purchase LPG in Australia.
LPG is supplied in gas bottles that are either exchanged or refilled on site by LPG tankers.
Large users may utilise bigger LPG storage tanks.
Propane is also frequently used in Autogas, alone or in a propane-butane mix.
LPG or propane gas go by a number of names in Australia including LPG, LPG gas, bottled gas, propane, BBQ gas, camping gas and LP gas.
However, no worries, as it’s all the same gas.
The chemical formula for Propane is C3H8. (Propane molecule model shown)
Isobutane Boiling Point
Isobutane boiling point is at -11.75°C, whereas butane boiling point is at -0.4°C.
This means you have a problem if you try to use pure isobutane when the temperature drops below -11.75°C but it is better than the butane boiling point (boiling point for butane) at -0.4°C.
Butane Boiling Point – Boiling Point for Butane
The butane boiling point (boiling point for butane) temperature is -0.4°C.
This is significantly higher than propane boiling point and can be problematic in colder climates.
Propane Boiling Point
The propane boiling point temperature is -42°C.
This propane boiling point temperature is sufficiently low that vaporisation can be achieved in almost all ambient temperature situations, outside of maybe the polar regions.
Liquid Propane or Propane Gas – Liquid Isobutane or Isobutane Gas – Liquid Butane or Butane Gas
| Liquid Propane or Gaseous Propane – Liquid Butane or Butane Gas | ||
|
LPG (1atm)
|
Liquid
|
Vapour (Gas)
|
|
Propane Boiling Point
|
< -42°C
|
≥ -42°C
|
| Isobutane Boiling Point |
< -11.75°C
|
≥ -11.75°C
|
|
Butane Boiling Point
|
< -0.4°C
|
≥ -0.4°C
|
Propane Gas, Butane Gas & Isobutane Gas Properties
This chart shows some of the physical property differences between propane, butane and isobutane gases.
You can refer back to the chart as we explain the importance of the numbers in the following topics…
| Gas Properties | Isobutane | Butane | Propane |
| Chemical Formula | C4H10 | C4H10 | C3H8 |
| Energy Content: MJ/m3 | 110.4 | 111.4 | 95.8 |
| Energy Content: MJ/kg | 45.59 | 47.39 | 49.58 |
| Energy Content: MJ/L | 25.0 | 27.5 | 25.3 |
| Boiling Temp: Cº | -11.75 | -0.4 | -42 |
| Pressure @ 21ºC: kPa | 310.9 | 215.1 | 858.7 |
| Flame Temp: Cº | 1975 | 1970 | 1967 |
| Expansion: m3/L | 0.234 | 0.235 | 0.270 |
| Gas Volume: m3/kg | 0.402 | 0.405 | 0.540 |
| Relative Density: H2O | 0.60 | 0.58 | 0.51 |
| Relative Density: air | 2.07 | 2.00 | 1.53 |
| L per kg | 1.669 | 1.724 | 1.96 |
| kg per L | 0.60 | 0.58 | 0.51 |
| Specific Gravity @ 25ºC | 2.06 | 2.07 | 1.55 |
| Density @ 15ºC: kg/m3 | 2.533 | 2.544 | 1.899 |
One Big Happy Family
Propane gas, butane gas, and isobutane gas are all hydrocarbon gases that fall under the broad label of “LPG”, as they are all liquefied petroleum gases.
Propane gas, butane gas and isobutane gas are a group of flammable hydrocarbon gases that are liquefied through pressurisation and commonly used as fuel.
Propane, butane and isobutane are also called Natural Gas Liquids (NGLs), along with ethane, pentane and pentanes plus.
Their common distinguishing characteristic is that propane gas, butane gas and isobutane gas can be compressed into liquid at relatively low pressures.
Propane, butane and isobutane are used as fuel in combustion, for heat generation, but there are also many other applications for LPG.
LPG or Propane – The Name Game
What LPG or propane are called is greatly dependent on what country you are in.
In Australia, we call it LPG but it is propane .
Autogas in Australia can be either pure propane or propane mixed with butane.
In New Zealand, LPG is almost always a propane and butane mix.
In the USA, they don’t generally use the term LPG.
They just call it “Propane”.
In some countries, like England, you can specifically buy propane or butane .
In other countries, they call it “GPL” or “GLP” instead of “LPG”, as the acronym is based on different languages and syntax.
For example, in French it is “gaz de pétrole liquéfié”, in Italian it is “gas di petrolio liquefatto” or in Spanish it is “gas licuado de petróleo”.
“LPG gasul” is used in the Phillipines.
Energy Content – Myths & Facts
I’ve seen any number of articles saying that butane has more energy content and is, therefore, more economical to use.
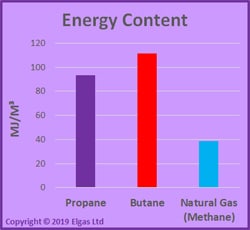 Not always true!
Not always true!
It actually depends on the unit of measure used for pricing.
If propane and butane are priced by volume — in litres — the butane has about 9% more energy content, with 27.5MJ/L versus 25.3 MJ/L for propane.
However, if propane and butane are sold by weight — in kilograms — then propane has about 5% more energy content, with 49.58MJ/kg versus 47.39 MJ/kg for butane.
Why?
Because propane has less density than butane, you get more litres per kilogram, with the difference more than offsetting the lower MJ/L energy content value.
Butane vs Propane vs Isobutane Temperature – Which is Hotter?
 The flame temperatures of butane vs propane vs isobutane temperature are virtually identical.
The flame temperatures of butane vs propane vs isobutane temperature are virtually identical.
Isobutane gas burns at 1975°C or 3587°F (isobutane flame temperature).
Butane gas burns at 1970°C or 3578°F (butane flame temperature).
Propane gas burns at 1967°C or 3573°F (propane flame temperature).
Butane Gas Combustion
Assuming complete combustion of butane gas, you get carbon dioxide and water:
2 C4H10 + 13 O2 → 8 CO2 + 10 H2O + Heat
However, with incomplete combustion you get carbon monoxide and water
2 C4H10 + 9 O2 → 8 CO + 10 H2O + Heat
This would typically occur if the ratio of oxygen to butane was insufficient.
Butane Boiling Point (Boiling Point for Butane) vs Propane Boiling Point: Turning from Liquid to Gas
Propane boiling point and butane boiling point (boiling point for butane) are different.
This is the temperature at which propane and butane go from liquid to gas (vapour).
 Propane boiling point is -42°C whereas butane boiling point (boiling point for butane) is -0.4°C.
Propane boiling point is -42°C whereas butane boiling point (boiling point for butane) is -0.4°C.
This means you have a huge problem if you try to use pure butane gas when the temperature drops below freezing.
No Boiling = No Vapourisation = No Gas
So, with butane only, you could find yourself with no gas for your heater and cooking appliances when it gets cold.
In some areas, LPG suppliers provide a mixture of propane and butane to address this problem.
This can work well when there are temperatures both below and above freezing.
However, the mixture in the cylinder can become butane rich if there is too much cold weather, with only the propane vapourising and being used.
Needless to say, propane is the preferred choice for cold weather climates.
What is Butane Commonly Used for?
The most common use of butane is as a heating fuel in a butane gas bottle or butane gas cylinder.
Butane can be used for cooking, hot water and space heating.
Butane is also frequently blended into autogas, to fuel vehicles.
There are also commercial and agricultural applications for butane, including the heating of greenhouses.
In non-fuel applications, butane is also commonly used as a propellant in aerosol products and as a refrigerant.
More Propellant Use for Butane Gas & Isobutane Gas
One of the other important differences between propane, butane and isobutane gases is vapour pressure.
Vapour pressure is the pressure exerted by the vapour (gas), in equilibrium with the liquid, against the walls of the cylinder or other closed container at a given temperature.
Propane has a much higher vapour pressure than either butane or isobutane.
Propane has approximately 4x the vapour pressure of butane and about 2.75x the vapour pressure of isobutane. (See properties chart above)
 Propane, butane and isobutane are all used as propellants in aerosol products, as they are naturally odourless, non-corrosive and non-toxic.
Propane, butane and isobutane are all used as propellants in aerosol products, as they are naturally odourless, non-corrosive and non-toxic.
For obvious reasons, no odourant is added to the LPG when used as a propellant.
Nobody wants a stinky hairspray!
Propane, butane and isobutane gases may be used individually or in combinations to achieve the desired pressure.
The lower pressures of butane and isobutane tend to be favoured for everything from deodorant to disposable cigarette lighters.
When the product label lists “hydrocarbon” as the propellant, it is often butane or isobutane.
Propane, butane and isobutane gases replaced chlorofluorocarbons (CFCs) as propellants about 30 years ago.
CFCs were banned because they damaged the ozone layer of the atmosphere.
Applications with Butane and Isobutane Refrigerant Gases
Propane, butane and isobutane gases are used in refrigeration but for different applications.
Propane, butane and isobutane have different refrigeration uses because of their different thermodynamic properties.
 Propane, butane and isobutane are used to replace the harmful CFC refrigerants, such as r12, r22, r134a.
Propane, butane and isobutane are used to replace the harmful CFC refrigerants, such as r12, r22, r134a.
High purity propane (>97.5%) is used as a refrigerant, known as propane refrigerant r290.
Both r600a isobutane refrigerant and n-Butane (r-600) are used as refrigerants.
Propane, butane and isobutane gases can also be mixed to achieve different properties, such as with propane refrigerant r-290a, a mixture of isobutane and propane.
Just as with propellants, propane, butane and isobutane gases also replaced chlorofluorocarbons (CFCs) as refrigerants, to preserve the ozone layer.
Improved Yields for Greenhouses with Butane
Butane gas is favoured, over propane, for use as fuel in greenhouses.
 Butane provides both heat and enriches the atmosphere with CO2, which aids in plant growth.
Butane provides both heat and enriches the atmosphere with CO2, which aids in plant growth.
While both propane and butane are environmentally friendly fuels, butane does have an extra carbon atom (C4H10 vs C3H8) that results in ⅓ more CO2 when burned.
Improved yields make butane the preferred choice for greenhouse use.
Butane vs Propane Availability Around the World
Propane is generally available in most countries.
Butane can be a bit harder to find in some areas.
Nevertheless, in many countries butane is available, either as pure butane or blended with propane .
Final Thoughts on Isobutane vs Butane vs Propane vs LPG
For many people, propane, butane and isobutane are indistinguishable LPG gases and never pose an issue.
However, for others propane, butane and isobutane provide the flexibility to use them for various specialised applications.
Either way, propane, butane and isobutane are exceptional energy products.
- Food: The Future of Food Starts Here: Paddock to Plate - February 25, 2026
- Bushfires & Gas Bottles: Safety Tips - January 9, 2026
- Sydney Gas Ban Explained: What It Means for New & Existing Homes - December 16, 2025


