The pressure in a gas cylinder is too high for most applications, at up to 2482 kPa. Gas regulators are connected to gas cylinder outlet valves to reduce the LPG-propane gas cylinder pressure to the much lower 2.75 kPa working pressure.
A gas cylinder works with pressure that is usually far in excess of what is required as the working pressure. There are specific gas regulators, by gas type, that are used to reduce the cylinder pressure down to the working pressure needed.
LPG is under pressure, as a liquid, and turns back into gas when you release some pressure. Gas cylinder pressure is too high and erratic to use, as temperature variation affects pressure. Gas cylinders require a gas regulator to reduce gas bottle pressure and provide a consistent working pressure.
An LP gas cylinder works by storing gas under pressure. To reduce the high pressure to a usable level, the gas is passed through a gas regulator to decrease the pressure to the correct level for the gas appliances. LPG is unique, as it liquefies under moderate pressure vs high pressure compressed gas cylinders.
An LPG gas cylinder works when the LPG, stored as a liquid under pressure, turns back into gas by releasing some of the pressure in the gas cylinder through use. The LPG gas cylinder-bottle works after being filled with the pressurised LPG. The LPG turns back into gas as it exits the LPG gas cylinder-bottle, then passing through a gas regulator, when the pressure is relieved by using some of the gas.
A propane tank works by storing the propane as a liquid, after it is filled under pressure, and changes back to gas when you relieve some of the pressure by using your BBQ or gas appliances. The gas is in the top of the LPG bottle & the liquid LPG at the bottom.
LPG is generally stored, as a liquid, in steel vessels ranging from small BBQ gas bottles to larger gas cylinders and storage tanks.
Gas in LPG Tanks
Gas in LPG tanks is LPG liquid under pressure, from 0 kPa at -42°C to 1794 kPa at 54°C, and turns back into gas when you release some of the pressure. The LPG (propane) exists as both liquid and vapour (gas) within the cylinder. See image below.
LPG Gas Cylinder Works (Propane Tank Works) with Liquid & Gas Inside
LPG gas cylinder works (propane tank works) with some of the LPG-propane at the top of the cylinder-tank is in gaseous form whilst it is liquid at the bottom. (See image)
LPG gases, including propane, can be compressed into liquid at relatively low pressures and is also referred to as natural gas liquids – NGL.
LPG-propane liquid boils and turns back into gas vapour when you release some of the pressure in the gas bottle by using gas.
Almost all of the uses for LPG involve the use of the gas vapour, not the liquefied gas.
LPG Gas Cylinder – Propane Tank – Cooking Gas Cylinder
LPG gas cylinders (propane tanks) may also be called “Propane Gas Bottles”, “LPG Bottles”, “LPG Gas Bottles” or just “Gas Bottle”.
LPG gas cylinders (propane tanks) work by containing both liquid and gas, as LPG liquefies under relatively low pressure.
An LPG gas cylinder (propane tank) is considered low pressure versus high pressure cylinders, as used with CNG.
LPG gas bottle sizes vary, based on application and demand.
A small LPG bottle is portable, as used in camping.
What are LPG Tanks Made of
LPG tanks are typically made of welded steel, aluminium or composites. Steel is by far the most common material, as it is the easiest to fabricate and it is a low cost material. Some cylinders are made of aluminium or composites to save on weight.
Aluminium is quite common for forklift cylinders, relating to safe lifting.
The use of aluminium keeps the weight down to allow for more gas whilst still staying within the safety weight limits.
The most recent innovation is the composite cylinder.
These are typically fiberglass with a high impact plastic outer shell.
Some have an inner lining of HDPE whilst other have a thin steel liner.
How LPG (Propane) Liquid Changes to Gas – Vaporisation
Did you know that every time you turn on one of your gas appliances, the LPG gas cylinder works (propane tank works) as your gas starts to boil? If you could see though the steel, you would also notice that it looks just like water boiling.
The big difference is that it happens at -42°C or -44°F.
This is vaporisation, which is how LPG – propane – goes from liquid to vapour (gas).
The LPG gas vapour is held in the top of the LPG bottle and the liquid LPG at the bottom, as shown in the accompanying image.
LPG (Propane) Boiling Point
Water boils at 100°C or 212°F, becoming a gas (steam). In contrast, LPG boils at -42°C or -44°F, becoming gas vapour.
LPG stays liquid because it is under pressure in an LPG cylinder (propane tank).
As a liquid, it looks a lot like water.
It is colourless and odourless in its natural state.
LPG (Propane) Vaporisation – How LPG Boils
When using LPG, you release it from the gas cylinder and it turns back into gas. From a practical point of view, it is used just like natural gas, although the LPG energy content is much higher. As it is portable, it can be used in vehicles as well as home heating, hot water and cooking.
To boil, the liquid LPG draws heat from the steel walls of the LPG gas cylinder (propane tank) which, in turn, works by getting heat from the ambient air.
LPG liquid boils and turns back into gas vapour when you release some of the pressure in the gas bottle (propane tank) by turning on your gas appliance.
As with water, the more heat that is applied, the more rapidly it boils, vaporising at a faster rate.
The vapour pressure in the gas bottle (propane tank) also increases with temperature, as explained below.
So, as the steel of the bottle draws heat from the ambient air heat, cold weather will slow down the rate of vaporisation.
Vaporisation also makes the gas bottle (propane tank) feel colder than the ambient temperature.
The gas bottle gets even colder when you are actually using the gas.
Under the right conditions, when you are using gas very rapidly, ice can even form on the gas bottle (propane tank)!
LPG Pressure
LPG pressure refers to the average force per unit of area that the LPG gas exerts on the inside walls of the LPG gas bottle (propane tank). LPG pressure varies with temperature. The LPG pressure goes from 0 kPa (0 PSIG or 0 bar) at -43ºC to 2482 kPa (360 PSIG or 24.8 bar) at 70ºC.
LPG is a liquefied gas. So, the pressure inside the cylinder will remain the same from full until the vaporistion of the last of the liquid LPG. Then the pressure will fall, with the use of the last of the LPG vapour.
LPG pressure measurement is in kilopascals (kPa), pounds per square inch (psi) or “Bar”.
LPG (Propane) Pressure Varies with Temperature
As previously mentioned, when LPG is stored in a gas bottle (propane tank), it is under pressure. The term “pressure” refers to the average force per unit of area that the gas exerts on the inside walls of the LPG bottle (propane tank).
(LPG Pressure-Temperature Chart shown)
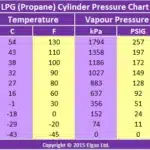
Pressure is measured in kilopascals (kPa) or pounds per square inch (psi).
“Bar” is yet another unit of measure for pressure.
1 Bar = 100 kPa, so it is metric based but not an SI unit of measure.
LPG pressure can vary greatly based on temperature, as shown in the chart.
The level of fill in the gas bottle comes into play when the LPG is being used, as it affects the rate of vaporisation.
How Much Pressure is in an LPG (Propane) Cylinder?
LPG is under pressure, as a liquid, and turns back into gas when you release some pressure. LPG gas cylinder-bottle works (propane tank works) with pressure of LPG (propane) going from 152 kPa (24 PSIG) at 0°C to 1794 kPa (257 PSIG) at 54°C.
The pressure drops to zero at -43ºC (which is just below the boiling point) and the pressure becomes greater at even higher temperatures.
Vaporisation Must Match Consumption When LPG Gas Cylinder Works (Propane Tank Works)
The amount of gas that the appliance or appliances are drawing from the gas bottles (propane tanks) must be matched by the rate of vaporisation when the LPG gas cylinder-bottle works (propane tank works).
Switching to a larger vessel can provide a higher rate of vaporisation.
Heat is absorbed through the vessel shell and into the liquid.
The larger the tank or the fuller the tank, the more gas that can be vaporised at a given temperature.
Vaporisation tables are used to match the required vaporisation rates to the corresponding vessel size.
Vaporisation tables show the maximum continuous vaporisation rates, in MJ/hr, at different ambient temperatures for each available vessel size.
LPG Gas Cylinder Works (Propane Tank Works) with LPG Cylinder Valves
All LPG cylinders work (propane tanks work) with some form of gas valve. A “POL” valve is the most common type of LPG valve in Australia, for most LPG cylinders from 4kg to 210kg.
The POL valve connection is notable for its reverse — left-handed — thread.
A pressure relief valve is incorporated within the POL valve, for safety.
Gas Line Connection
The gas line pigtail or regulator screws into the large female threaded opening on the side of the valve.
It is unique in that it has a left-handed or reverse thread. So, to tighten it, you turn the connector anti-clockwise.
Tightening is achieved either with a wrench or by turning a hand wheel.
You should always do a soapy water leak test after every reconnection.
LPG Gas Cylinder Works (Propane Tank Works) by Turning the Gas Valve Hand Wheel
The LPG gas cylinder works (propane tank works) by turning the hand wheel anti-clockwise to start the flow of gas, but you should avoid turning it hard to the open stop.
Located on the top of the POL valve, the gas valve hand wheel controls the flow of gas, once the connection is secure.
Opening the valve 2 or 3 turns is all you need.
You shut the gas off by turning the hand wheel clockwise, tightening firmly by hand only.
CAUTION: Never open the valve when unattached
Pressure Relief Valve
The pressure relief valve is the single most critical safety feature in how an LPG cylinder-bottle works (propane tank works).
It is incorporated within the POL valve and appears as the protrusion opposite the main connection.
It usually incorporates some kind of plastic dust cover that should be left in place.
Pressure relief valves are designed to relieve excess pressure that might result from overfilling or exposure to excessive heat or fire.
The function of a pressure relief valve is to keep a cylinder from rupturing in the unlikely event of excessive pressure build-up.
The pressure relief valves are held in the closed position by the force of a powerful spring inside.
As long as the pressure is less than that of the spring, the valve will remain closed.
LPG Gas Cylinder Works (Propane Tank Works) by Venting of Gas
If the pressure rises beyond safe levels, the LPG gas cylinder works (propane tank works) when the pressure relief valve opens to vent the excess pressure.
If this happens, you may hear a hissing sound and see cold gas vapour being released.
Once sufficient pressure is released, the valve closes.
If this ever happens, just stay clear of the area and let the gas dissipate.
Having the pressure relief valve releasing gas is a rare event. Most people will never experience venting.
You should also call your LPG supplier, from a safe location, and advise them that your gas cylinder is venting gas.
Do not use your mobile phone, any electrical devices or other ignition sources near a venting gas cylinder.
Final Thoughts
An LPG gas cylinder-bottle works (propane tank works) unnoticed after evolving over many decades into a very safe storage and gas delivery system.
They are also easy to operate, requiring little attention until they need refilling.
