Propane, Propane Gas & Liquid Propane Gas | Propane Gas Bottle – Propane Tank | Is Propane the Same as LPG
Propane is a clean-burning fuel gas widely used in Australia for heating, cooking, and powering home appliances.
It is a byproduct of natural gas processing and oil refining, stored in liquid form and released as gas. In Australia, propane is the primary component of LPG.
What is Propane Gas? What is Liquid Propane? | Propane Tank – Propane Gas Bottle
What is liquid propane? Propane or propane gas is a flammable hydrocarbon gas that is converted into liquid propane gas through pressurisation in a propane tank with propane (propane gas bottle) and commonly used for fuel in heating, cooking, hot water and vehicles.
A propane gas bottle or propane tank with propane liquid is the preferred storage method for liquid propane gas..
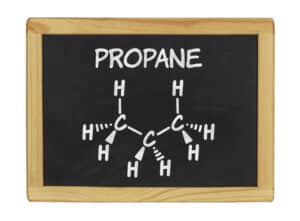
The propane formula or propane chemical formula (formula for propane) is C3H8 meaning a propane molecule has 3 carbon and 8 hydrogen atoms, the propane structure formula.
Propane (propane gas) or liquid propane gas is a co-product of natural gas processing and a derivative of crude oil refining.
Is Propane the Same as LPG – Is LP Gas the Same as Propane –
LPG vs. Propane – What’s the Difference?
In Australia, LPG refers to propane. In other countries, LPG may be a blend of propane and butane.
If you’re buying LPG in Australia for household use, you are using propane.
Is Liquid Petroleum Gas the Same as Propane?
LPG or propane (propane gas)? In Australia, liquid petroleum gas (LPG) is the same as propane (propane gas).
Propane is liquid petroleum gas (LPG) but not all liquid petroleum gas (LP gas) is propane.
So, regarding liquified petroleum gas vs propane, is LP gas same as propane (propane gas) the answer is “it depends”.
Liquid petroleum gas (LPG) also includes butane (n-butane) and isobutane (i-butane), as well as mixtures of the three LPG gases.
These gases are also referred to as natural gas liquids – NGL, as they are stripped from natural gas during processing.
What is Liquid Propane? Liquid Propane Gas
 What is liquid propane or liquid propane gas?
What is liquid propane or liquid propane gas?
Liquid propane (liquid propane gas) is propane under pressure in a propane tank (propane gas bottle), as propane liquefies under pressure.
Propane gas is stored under pressure as liquid propane (liquid propane gas) in a propane gas bottle or propane tank with propane (propane gas).
When the pressure is released from the propane tank (propane gas bottle), the liquid propane gas turns into propane gas.
Propane Formula – Propane Chemical Formula – Formula for Propane
The propane formula or propane chemical formula (formula for propane) for the propane molecule is C3H8, the propane structure formula, indicating that a propane molecule has 3 carbon and 8 hydrogen atoms.
Propane Boiling Point
Liquid propane gas turns into propane gas at -42 °C or -44 °F, which is the propane boiling point.
Conversely, propane gas turns into liquid propane gas when it drops below -42 °C or -44 °F, the propane boiling point in a propane tank (propane gas bottle).
Is LPG Propane – Is Propane Gas LPG – Propane Gas vs LP Gas
Propane is LPG but not all LPG is propane.
Propane gas is one of the gases that fits the definition of LPG – Liquefied Petroleum Gas.
Regarding propane gas vs LP gas, propane gas is the same as LP gas in Australia and the USA.
LPG or propane gas is stored as a liquid in a small propane tank (propane gas bottle) or larger propane tank, compressed into liquid at relatively low pressures.
The common uses for a propane gas bottle is as fuel gas for home cooking, heating, hot water and vehicles.
Propane gas can also be used for refrigerants, aerosol propellants and petrochemical feedstock.
LPG or propane gas comes from natural gas processing and crude oil refining and stored in a propane tank (propane gas bottle).
Propane Gas & Liquid Propane Gas Physical Properties
Propane Boiling Point, Energy Content, Flame Temperature, Chemical Formula, Density, Specific Gravity and more…
Propane Gas & Liquid Propane Gas Properties Chart
Propane Gas & Liquid Propane Gas Properties |
|
| Propane Properties | Propane |
| Propane Formula | C3H8 |
| Propane Energy Content: MJ/m3 | 95.8 |
| Propane Energy Content: MJ/kg | 49.58 |
| Propane Energy Content: MJ/L | 25.3 |
| Propane Boiling Temp: Cº | -42 |
| Propane Pressure @ 21ºC: kPa | 858.7 |
| Propane Flame Temp: Cº | 1967 |
| Propane Expansion: m3/L | 0.270 |
| Propane Gas Volume: m3/kg | 0.540 |
| Propane Relative Density: H2O | 0.51 |
| Propane Relative Density: air | 1.53 |
| Propane kg per L | 0.51 |
| Propane L per kg | 1.96 |
| Propane Specific Gravity @ 25ºC | 1.55 |
| Propane Density @ 15ºC: kg/m3 | 1.899 |
Note: Some propane gas and liquid propane gas numbers have been rounded.
Where Does Liquid Propane Gas Come From?
Liquid propane gas comes from natural gas wells and oil wells.
Liquid propane gas is a fossil fuel that does not occur in isolation.
Propane (propane gas) is found naturally in combination with other hydrocarbons.
Liquid propane gas is a co-product of natural gas processing and crude oil refining.
Propane gas is isolated, liquefied through pressurisation into liquid propane gas and stored in pressure vessels, like a propane tank (propane gas bottle), for easy storage, shipping and distribution.
How is Propane Gas Made? Propane Tank with Propane – Propane Gas Bottle
LPG or propane gas is made during natural gas processing and oil refining.
It is separated from the unprocessed natural gas using refrigeration.
Propane gas is extracted from heated crude oil using a distillation tower.
Propane gas is then pressurised and stored as liquid propane gas in a propane gas bottle or a propane tank with propane.
Propane turns into propane gas when it reaches -42 °C or -44 °F, the propane boiling point in propane tank (propane gas bottle).
How is Propane Gas Made from Natural Gas?
LPG or propane gas isn’t so much made from natural gas as it is separated from natural gas.
Propane gas is separated from the raw natural gas stream by ‘stripper plants’ that literally strip the propane gas from the raw natural gas stream.
It is important to understand that “raw natural gas”, as it leaves the gas well, contains other gases (including propane gas) and impurities that need to be processed out to obtain the nearly pure methane gas that we refer to as “refined natural gas” or just “natural gas”.
Applications for Propane Gas
 Propane is used in transportation as a fuel, typically called Autogas, for vehicles in general and in low emission applications.
Propane is used in transportation as a fuel, typically called Autogas, for vehicles in general and in low emission applications.- The hospitality industry – hotels, restaurants, pubs and clubs – use propane gas for heating, cooking and hot water. A propane tank with propane (propane gas bottle) is used for storage.
- Liquid propane gas is used in a propane tank with propane (propane gas bottle) for forklifts.
- Chicken farmers use propane gas to keep the chicks warm.
- Farm uses include mounting a propane tank with propane (propane gas bottle) on a tractor for flame weeding.
- A stationary propane tank (propane gas bottle) to fuel crop drying and irrigation pumps.
- Auto body repair shops use propane gas for the oven to cure painted cars. A propane tank with propane (propane gas bottle) is used for storage.
- Refrigeration manufacturers use propane gas for a refrigerant gas.
- Many companies use propane gas to fuel boilers for a multitude of purposes. And the list for uses just goes on and on.
Propane Safety: Is Propane Safe to Use?
Is Propane Safe?
Propane is highly flammable and must be stored and handled properly.
- Odourised for safety: Ethyl mercaptan is added to detect leaks.
- Tank filling to 80%: Propane bottles are never filled to 100% to prevent overpressure.
- Use outdoors or in ventilated areas.
Always follow the manufacturer’s safety instructions and have your system inspected regularly.
An odorant, typically ethyl mercaptan, is added to propane for safety reasons, as the smell it makes it easier to detect leaks.
Following these tips to help ensure your propane gas bottle (propane tank) is used, stored and transported safely.
- A propane gas bottle (propane gas cylinder) should never be laid down. Always keep a propane gas bottle (propane tank) upright and secured for storage and transportation.
- Make sure gas appliances are only operated with adequate ventilation.
- Never tamper with a propane gas bottle or gas valve.
- If excess force is needed to open or close the propane gas bottle valve, or if the valve becomes damaged, notify or return to the gas supplier.
- Keep a propane gas bottle (propane tank) away from sources of ignition or heat at all times.
- If a propane gas bottle (propane tank) valve develops a leak, make sure the valve is closed and move the propane gas bottle to a safe location at least 20 metres from any ignition source. When empty, return to the supplier with attached explanation.
- Only use propane gas with propane appliances and never with natural gas appliances.
- All gas fitting work is must be performed by a licensed gas fitter.
- In the event of a fire, dry chemical fire extinguishers may be used for propane gas fires.
What Propane Is Used For
Common Uses of Propane:
- Heating homes, pools, and commercial space
- Cooking on BBQs, gas stoves, and camp cookers
- Powering gas dryers, water heaters, and generators
- Fuel for forklifts, RVs, and backup generators
- Industrial welding and cutting applications
 LPG – propane gas is used in a propane tank (propane gas bottle) as fuel in homes, business, industrial and agricultural, primarily for propane gas space heating, water heating, BBQ, and cooking.
LPG – propane gas is used in a propane tank (propane gas bottle) as fuel in homes, business, industrial and agricultural, primarily for propane gas space heating, water heating, BBQ, and cooking.
Liquid propane gas is also used in transportation as fuel (Autogas) for internal combustion engine applications including cars, forklifts, buses, irrigation pumps, and fleet vehicles with a propane tank (propane gas bottle).
LPG – propane gas uses in everyday home life including a propane tank with propane (propane gas bottle) for home heating, cooking, hot water heaters, gas fireplaces and clothes drying.
Outdoor home uses include a propane tank with propane (propane gas bottle) for patio heaters, pool heaters, backup generators, and as BBQ fuel.
In addition, propane gas is used for propellant, refrigerant gas and petrochemical feedstock applications.
Propane gas does get used in leisure time activities including a propane tank with propane (propane gas bottle) for caravans, boats, recreational vehicles, hot air balloons and camping.
LPG – propane gas is also used for fuel for many commercial and agricultural heat applications, including propane gas steam boilers, kilns, ovens and forklifts
Crop and produce drying, heating greenhouses, propane gas hot water for dairies, irrigation pumps and heating animal enclosures are just some of the agricultural applications for propane gas.
There are also many, many more propane gas applications, including propane gas power generation and the hospitality industry. A propane tank with propane (propane gas bottle) is used for storage.
Propane Gas Used as a Fuel Gas in Transportation and Stationary Applications
LPG – propane gas is used as a fuel because of its portability, in a propane tank (propane gas bottle), and high energy density.
Applications consist of vehicles and stationary uses including generators, ovens, kilns, crematory and other heat applications.
In addition, propane gas only requires modest pressure for liquefication keeping the propane tank weight down.
Propane turns into propane gas when it reaches -42 °C or -44 °F, the propane boiling point in a propane tank (propane gas bottle).
LPG or Propane? Goes by Many Names
Is it LPG or propane?
In Australia, propane gas has many names.
It is also called LPG, LPG Gas, LP Gas, BBQ Gas or Autogas.
In the USA it is just called Propane.
In the UK, it is referred to as either LPG or propane.
Propane Tank: How a Propane Tank with Propane Works – Vaporisation in Propane Gas Bottle
Propane Boiling Point at -42 °C or -44 °F
 A propane tank with propane is liquid propane gas stored under pressure, as a liquid, in propane tank (propane gas bottle).
A propane tank with propane is liquid propane gas stored under pressure, as a liquid, in propane tank (propane gas bottle).
Liquid propane gas turns back into gas vapour when you release some of the pressure in the propane tank (propane gas bottle) by turning on your appliance through a process called vaporisation.
Propane boils when it reaches -42 °C or -44 °F, the propane boiling point in a propane tank (propane gas bottle).
To reach propane boiling point, the liquid propane gas draws heat from the steel walls of the propane tank (propane gas bottle) which, in turn, get heat from the ambient air.
Almost all of the uses for propane gas involve the use of the gas vapour, not the liquid propane gas.
There are special propane tank (propane gas bottle) types for liquid withdrawal.
Propane Gas Composition: Propane Formula, Propane Chemical Formula or Propane Structure Formula
 The propane formula or propane chemical formula (formula for propane) is C3H8, the propane structure formula.
The propane formula or propane chemical formula (formula for propane) is C3H8, the propane structure formula.
Propane molecule structure model shown.
The propane formula or propane chemical formula (propane structure formula) describes a hydrocarbon gas with 3 carbon and 8 hydrogen atoms in a propane molecule.
Propane gas is not made or manufactured, it is found naturally in combination with other hydrocarbons.
It is produced or “made” during natural gas processing and crude oil refining and stored in a propane tank (propane gas bottle).
Both processes begin by drilling oil wells.
Propane gas does not occur naturally in isolation.
LPG or Propane Gas Heating Systems for Homes
LPG or propane gas heating systems for home are an energy efficient way to keep warm and cozy.
Propane gas heating systems works well for large home spaces.
A propane tank with propane (propane gas bottle) is used for storage.
LPG or propane gas heating systems are synonymous with fast home heating and real warmth.
Propane gas heating system sub-types include radiant, convectors, radiant-convectors, flued radiant, freestanding fireplaces, fireplace inserts, inbuilt fireplace gas heaters and power flued gas heaters for the home.
With a wide range of propane gas heating appliances systems to choose from, there is a model that is just right for your home.
Storage in a Propane Gas Bottle – Propane Tank with Propane
An LPG or propane tank with propane gas (propane gas bottle) is typically a steel vessel for storing the gas.
Homes and small businesses typically use either a 45kg propane gas bottle or the larger 90kg or 210kg propane tank with propane.
BBQ propane tanks with propane gas comes in 9kg and 4kg propane tank (propane gas bottle) sizes.
 High volume commercial users have the larger propane tank sizes, with some as large as 100 tonne capacity.
High volume commercial users have the larger propane tank sizes, with some as large as 100 tonne capacity.
A propane tank (propane gas bottle) contains liquid propane gas and propane gas, as propane liquefies under relatively low pressure.
Propane turns into propane gas when it reaches -42 °C or -44 °F, the propane boiling point in a propane tank (propane bottles).
Propane tanks are considered low pressure versus high pressure cylinders, as used with CNG.
Propane tanks sizes and volumes vary, based on application and demand.
A small propane tank (propane gas bottle) is portable, as used in camping.
You Can Bury a Propane Tank
You can bury a propane tank with propane (propane gas bottle) as long as it was made for burying.
A buried liquid propane tank (propane gas bottle) needs anti-corrosive coatings and cathode protection.
An underground propane tank (propane gas bottle) is tanker filled by hose.
The burying of a liquid propane tank (propane gas bottle) should be handled by an experienced professional company and minimum and maximum distances from the propane tank (propane gas bottle) to your home or business apply.
A buried propane tank (propane gas bottle) must also be safely away from any ignition source.
Propane Tank or Propane Gas Bottle Installation Distance Requirements & Regulations
Residential LPG or propane tank regulations and propane tank (propane gas bottle) distance requirements are important for a safe installation.
The installation distance requirements can vary based on the size of the propane tank (propane gas bottle).
The typical minimum distance is 10 feet or 3 meters for a propane tank with propane (propane gas bottle), including ignition sources and building openings in all directions.
Propane Gas Pressure Regulators
 Propane turns into propane gas when it reaches -42 °C or -44 °F, the propane boiling point in a propane tank (propane gas bottle).
Propane turns into propane gas when it reaches -42 °C or -44 °F, the propane boiling point in a propane tank (propane gas bottle).
A propane gas regulator is used to control the pressure of the propane gas delivered to the appliance, from the propane tank (propane gas bottle).
Liquid propane gas pressure regulators automatically modulate high pressure propane gas down to a maximum pre-determined limit.
The pressure within a propane tank (propane gas bottle) can be 800-900kPa which is regulated down to the 2.75kPa typically required.
Liquid propane gas pressure regulators are usually factory pre-set.
Is Propane Gas Explosive? Can a Propane Tank Explode?
A propane tank (propane gas bottle) explosion or blast is actually quite rare.
Propane tank (propane gas bottle) can be explosive but not easily or often.
Even trying to create a propane tank (propane gas bottle) explosion intentionally is very challenging.
Propane gas is explosive only under the right circumstances.
The propane gas can be explosive if it is within the limits of flammability, between 2.15% and 9.6% of the total propane gas/air mixture.
For the propane gas to be explosive, it must also collect in a confined space for an explosion to occur.
Odourant is Added to Liquid Propane Gas for Safety
 In its natural state, LPG or propane gas is an odourless gas.
In its natural state, LPG or propane gas is an odourless gas.
The distinctive smell that people associate with propane gas is actually added to liquid propane gas as a safety measure.
Without the addition of an odourant, leaking propane gas could collect without being detected.
Avoid Direct Contact with Liquid Propane Gas – Cold Burns
Caution should always be used to avoid direct exposure, as liquid propane gas is cold enough to cause severe cold burns on exposed skin.
Specific Gravity of Propane Gas and Liquid Propane Gas – Density
 Unlike water, 1 kilogram of liquid propane gas does NOT equal 1 litre of liquid propane gas.
Unlike water, 1 kilogram of liquid propane gas does NOT equal 1 litre of liquid propane gas.
Liquid propane gas density or specific gravity is about half that of water, at 0.51.
1kg of liquid propane gas has a volume of 1.96L.
Conversely, 1L of liquid propane gas weighs 0.51kg.
1lb of liquid propane gas @ 60ºF has a volume of 0.24 US gallons.
Conversely, 1 US gallon of liquid propane gas @ 60ºF only weighs 4.23lbs, instead of the 8.34lbs it would weigh if it was water.
Propane Gaseous Expansion at Propane Boiling Point
 Liquid propane gas expands to 270 times the volume when it reaches -42 °C or -44 °F, the propane boiling point in a propane tank (propane gas bottle) and is released from the propane tank (propane gas bottle).
Liquid propane gas expands to 270 times the volume when it reaches -42 °C or -44 °F, the propane boiling point in a propane tank (propane gas bottle) and is released from the propane tank (propane gas bottle).
So, 1L of liquid propane gas equals 270L of gaseous propane.
As there are 1000L in a cubic meter (M3), 1L of liquid propane gas expands to 0.27M3.
1 US gallon of liquid propane gas equals 36.38ft3 of gaseous propane
Energy Content of LPG or Propane gas
LPG or liquid propane gas contains approximately 25MJ per litre.
This also converts to 6.9kWh.
More LPG or propane gas energy content facts:
1 US gallon of liquid propane gas = 91,502 BTU @ 60ºF
1ft3 propane gas = 2,488 BTU of Gas @ 60ºF
1 US gallon liquid propane gas = 1.1 therm
1 therm = 100,000 BTU
1 watt = 3.41214 BTU/h
Propane Formula or Propane Chemical Formula for Combustion
Propane formula or propane chemical formula (formula for propane) for combustion in words:
Propane gas + Oxygen → Carbon Dioxide + Water + Heat
Propane formula for combustion expressed with molecular formulas:
(C3H8 + 5 O2 → 3 CO2 + 4 H2O + Heat)
In the presence of sufficient oxygen, propane gas burns to form water vapour and carbon dioxide, as well as heat.
If not enough oxygen is present for complete combustion, incomplete combustion occurs with water, carbon monoxide, and carbon dioxide being produced.
LPG or Propane Gas Flame Temperature
 An LPG or propane gas flame burns at 1980ºC (3596ºF).
An LPG or propane gas flame burns at 1980ºC (3596ºF).
When it is burning properly, the propane gas flame is blue.
A yellow or red flame is usually indicative of incomplete combustion.
LPG or Propane Gas Limits of Flammability
LPG or propane gas lower and upper limits of flammability are the percentages of propane gas that must be present in an propane/air mixture.
This means that between 2.15% and 9.6% of the total propane gas/air mixture must be propane gas in order for it to be combustible.
LPG or Propane Flash Point
The flash point of LPG or propane is -104ºC (-155.2ºF).
This is the minimum temperature at which propane will burn on its own after having been ignited.
Below this temperature, propane will stop burning on its own.
However, if a source of continuous ignition is present, propane will burn below -104°C.
Dissipation of Propane Gas
Propane gas is heavier than air and will sink to and collect at the lowest point.
If propane gas is vented to the outside air, it will quickly dissipate with the slightest movement of air.
Conversely, if propane gas is vented into a sealed structure, with no air movement, the propane gas will collect on the floor and rise vertically as more propane gas is vented into the structure.
Propane Gas Vapour vs. Liquid Propane Gas Use
Propane can be supplied from a propane tank for either liquid propane gas or propane gas vapour.
The difference is in the extraction from propane tank or vessel in which it is supplied.
Most propane gas applications use vapour.
Appliances such as water heaters, room heaters and cookers all use propane gas vapour.
If these appliances were to have liquid propane gas flow to their burners, the result could possibly be a fire or similar safety hazard.
This is why a propane tank with propane should always be kept upright, so that only propane gas vapour is released.
A special liquid withdrawal propane tank with propane is used for the special applications, where required.
- LPG vs Natural Gas – Difference Between LPG Gas and Natural Gas – What is LPG - May 19, 2025
- Propane, Propane Gas & Liquid Propane Gas | Propane Gas Bottle – Propane Tank | Is Propane the Same as LPG - May 19, 2025
- LPG – LPG in Gas – What is LPG: What is Liquefied Petroleum Gas – LPG Gas - May 18, 2025
Steve Reynolds
Technical Consultant
Steve Reynolds is a leading expert in the LPG industry with over 22 years of experience. As part of the national management team at ELGAS, Steve ensures the safe and efficient storage, handling, and transportation of LPG. He serves as the lead investigator for incidents and collaborates with authorities on industry developments.
Steve is a technical advisor to Standards Australia and Gas Energy Australia (GEA), and an active member of the World LPG Association (WLPGA), contributing to global standards and technical reviews. He holds a BSc. (Hons) in Industrial Chemistry from UNSW and has held senior safety and technical roles at ELGAS, making him a trusted authority in LPG safety and standards.
