LPG Pressure – How Much Pressure in LPG Cylinder – LPG Pressure Relief Valve – Gas Cylinder Pressure
The LPG cylinder pressure in bar goes from 0 bar at -43ºC to 24.8 bar at 70ºC. Most pressure relief valves are set at around 25 bar, so that is about as high as LPG cylinder pressure will go. LPG pressure and how much pressure in LPG cylinder is dependent upon temperature. The pressure of LPG (propane) goes from 0 bar at -43ºC to 152 kPa (24 PSIG or 1.5 bar) at 0ºC to 2482 kPa (360 PSIG or 24.8 bar) at 70ºC. The LPG – propane – exists as both liquid and vapour (gas) within the cylinder.
The LPG gas pressure at which liquid LPG turns to gas is the vapour pressure. This varies based on temperature and the propane – butane mix. The pressure of LPG (propane) goes from 0 bar at -43ºC to 152 kPa (24 PSIG or 1.5 bar) at 0ºC to 2482 kPa (360 PSIG or 24.8 bar) at 70ºC.
The higher the temperature, the higher the pressure of the LPG within the cylinder.
LPG gas cylinder-bottle pressure all depends on the temperature. At 100°F, a 20lb propane tank has 172 PSIG of pressure. pressure is in a 100 lb propane tank would also be 172 PSIG, as the size of the propane tank doesn’t matter, only the temperature.
Similarly, a 9kg gas bottle has 1183kPa at 38°C.
LPG cylinder pressure in bar goes from 0 bar at -43ºC to 24.8 bar at 70ºC. The higher the temperature, the higher the LPG pressure or LPG gas cylinder pressure. The LPG pressure relief valve is a safety device that prevents excessive pressure in LPG cylinder by releasing some of the gas if the LPG pressure gets too high.
First, some background regarding how much LPG pressure and LPG gas cylinder pressure (LPG gas bottle pressure):
• LPG gas bottle pressure or larger vessel pressure, is dependent upon the temperature of the vessel.
• The LPG – propane – exists as both liquid and vapour (gas) within the cylinder and is also referred to as natural gas liquids – NGL.
• The term “pressure” refers to the average force per unit of area that the gas exerts on the inside walls of the LPG gas cylinder.
LPG Gas Pressure – LPG Pressure
LPG gas pressure refers to the average force per unit of area that the LPG gas exerts on the inside walls of the LPG gas bottle. LPG gas bottle pressure (LPG gas cylinder pressure) varies with temperature. The LPG gas pressure goes from 0 kPa (0 PSIG or 0 bar) at -43ºC to 2482 kPa (360 PSIG or 24.8 bar) at 70ºC.
LPG is a liquefied gas. So, the LPG pressure inside the cylinder will remain the same from full until the vaporisation of the last of the liquid LPG. Then the LPG pressure will fall, with the use of the last of the LPG vapour.
LPG pressure measurement is in kilopascals (kPa), pounds per square inch (psi) or “Bar”.
Propane Tank Pressure vs Temperature
Above -42°C (-43.6°F) and at NTP – normal temperature and pressure – propane is gaseous. Under pressure or at lower temperatures propane is a liquid. Propane is naturally odourless and colourless and looks like water.
LPG Gas Cylinder Pressure (LPG gas bottle pressure) – Temperature Chart
How much LPG pressure in LPG gas cylinder? As temperature rises in an LPG gas cylinder-bottle, so does the LPG gas cylinder pressure (LPG gas bottle pressure).
For example, LPG gas cylinder pressure (LPG gas bottle pressure) is 0 kPa (0 PSIG or 0 bar) at -43ºC and goes up to 2482 kPa (360 PSIG or 24.8 bar) at 70ºC.
The LPG is both liquid and vapour (gas) inside the LPG gas cylinder.
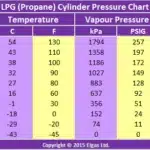
LPG (propane) gas cylinder-bottle pressure chart
| Temp | Temp | Pressure | Pressure | Pressure |
| ºC | ºF | kPa | PSIG | Bar |
| 70 | 158 | 2482 | 360 | 24.8 |
| 60 | 140 | 2013 | 292 | 20.1 |
| 54 | 130 | 1794 | 257 | 17.9 |
| 43 | 110 | 1358 | 197 | 13.6 |
| 36 | 100 | 1186 | 172 | 11.9 |
| 32 | 90 | 1027 | 149 | 10.3 |
| 27 | 80 | 883 | 128 | 8.8 |
| 16 | 60 | 637 | 92 | 6.4 |
| -1 | 30 | 356 | 51 | 3.6 |
| -18 | 0 | 152 | 24 | 1.5 |
| -29 | -20 | 74 | 11 | 0.7 |
| -43 | -45 | 0 | 0 | 0 |
Note: Some numbers have been rounded.
For LPG gas cylinder-bottle pressure see chart above.
Units of Measure for LPG Gas Pressure – LPG Gas Cylinder-Bottle Pressure in Bar, kPa & PSI
There are 3 commonly used units of measure for LPG gas cylinder pressure (LPG gas bottle pressure): PSIG, kPa & bar.
PSIG is LPG gas pressure in pounds per square inch gauge. PSIA is LPG gas pressure in pounds per square inch absolute.
1 atm = 14.7 psia = 0 psig = 101.325 kPa
kPa, or kilopascal, is a metric unit of pressure and part of the International System of Units (SI).
LPG Cylinder Pressure in Bar
The LPG cylinder pressure in bar goes from 0 bar at -43ºC to 24.8 bar at 70ºC. LPG cylinder pressure in bar varies by temperature. The higher the temperature, the higher the LPG cylinder pressure in bar.
LPG cylinder pressure in bar is also a metric unit of LPG gas pressure, but is a non-SI unit.
1 bar = 100 kPa = 0.987 atm = 14.5038 psia
Propane Gas Bottle Pressure
Propane gas bottle pressure in bar goes from 0 bar at -43ºC to 24.8 bar at 70ºC. Propane gas bottle pressure is dependent upon temperature. The pressure of propane goes from 152 kPa (24 PSIG or 1.5 bar) at 0ºC to 2482 kPa (360 PSIG or 24.8 bar) at 70ºC.
Propane Tank Pressure – LPG Gas Cylinder Pressure (LPG gas bottle pressure) in PSI & kPa
Propane tank pressure in PSI (Pounds per Square Inch) or LPG gas cylinder pressure (LPG gas bottle pressure) in PSI or kPa pressure all depends on the temperature. The higher the temperature, the higher the propane tank pressure in PSI (or kPa) – LPG gas cylinder pressure (LPG gas bottle pressure).
A 20lb (9kg) propane tank has the same propane tank pressure in PSI as larger propane tanks at a given temperature.
At 100°F, a propane tank of any size, including a 20lb propane tank (LPG gas cylinder-bottle), has a propane tank pressure in PSI of 172 PSIG.
At 130°F, propane tank pressure (LPG gas cylinder-bottle pressure) of any size has a propane tank pressure in PSI of 257 PSIG.
PSIG (pounds per square inch gauge) is the gauge LPG gas pressure relative to atmospheric pressure.
There is also PSIA – pounds per square inch absolute – for LPG gas pressure relative to a vacuum rather than the ambient atmospheric pressure.
In What State is the LPG Gas Inside the Cylinder?
LPG is liquid in a cylinder with an area at the top of the cylinder where it turns into LPG vapour under pressure. The LPG gas vapour pressure is held in the top of the bottle and the liquid LPG at the bottom, as shown in the accompanying image.
LPG cylinders are typically filled to 80%, meaning 80% liquid and 20% vapour.
This percentage changes, with the liquid LPG decreasing as you consume the gas.

Pressure Relief Valves for LPG Tanks – Safety Relief Valve Prevents Over-Pressure – Propane Tank Safety Relief Valve
Pressure relief valves (propane tank safety relief valve) for LPG-propane tanks are required by law. They are pressure relief devices that release gas when there is excess pressure. This release of pressure is to prevent the LPG tanks rupturing from excessive pressure, preventing a possible BLEVE.
Pressure relief valves for LPG tanks are safety devices that prevents excessive pressure or over-pressure in the cylinder by releasing some of the gas if the LPG pressure gets too high. If the LPG cylinder is exposed to excessive heat the pressure will rise. If the pressure gets too high, the LPG pressure relief valve allows the gas to vent, keeping the LPG cylinder pressure within safe limits. The typical LPG pressure relief valve setting is 2585 kPa or 375 PSIG.
So, the LPG cylinder or LPG tank pressure would never exceed this, as the valve would open and lets some gas escape, limiting the LPG pressure inside the tank and never even approaching the LPG tank maximum pressure limit.
LPG pressure relief valves are incorporated into the main LPG valves. It’s actually a safety relief valve within a valve.
The LPG pressure relief valve may also be known as a propane pressure relief valve or safety relief valve.
The LPG pressure relief valve is integrated into the main gas valve on the bottle, as shown in the accompanying picture. On larger vessels it may be a separate LPG pressure relief valve.
The LPG pressure relief valve eliminates the possibility that the cylinder will rupture or explode.
The worst thing that can happen is the venting gas ignites and you have a plume of flame.
This flame will self-extinguish after the LPG gas cylinder pressure (LPG gas bottle pressure) drops to a safe level or when the cylinder runs out of gas.
This is why you always want to use your BBQ outdoors and away from your home or other flammable materials.
LPG Boiling Point
LPG (Propane) Vaporisation: Did you know that every time you turn on one of your gas appliances, the LPG in your gas bottles starts to boil creating the LPG gas pressure?
If you could see through the steel, you would also notice that it looks just like water boiling…
Water boils at 100°C, becoming a gas (steam).
In contrast, LPG boils at -42°C becoming gas vapour.
The process of going from liquid to vapour (gas) is called vaporisation.
LPG stays liquid because it is under LPG pressure in a gas cylinder.
As a liquid, it looks a lot like water.
It is colourless and odourless in its natural state.
For more info on LPG, see LPG Gas Physical Properties
Liquefaction: The Conversion of LPG Vapour to LPG Liquid – Vapour Pressure
The conversion of LPG vapour to LPG liquid is called liquefaction, and depends on temperature and LPG pressure of the vapour. The higher the temperature of the vapour, the higher the LPG vapour pressure needed to convert the vapour to liquid.
For propane vapour at 20°C it must be pressurised to about 836 kPa to see it liquefy. At 50°C, about 1713 kPa LPG vapour pressure is required. The lower the temperature, the easier it is to liquefy the vapour.
For butane vapour at 20°C it must be pressurised to about 115 kPa to see it liquefy. At 50°C, about 510 kPa LPG vapour pressure is required.
For mixtures of propane and butane, the liquefaction conditions also depend on the composition of the mix, as well as the temperature and LPG vapour pressures.
Propane Tank Max Pressure Rating – LPG Cylinder Pressure Rating
The propane tank max pressure rating is the design LPG pressure limit of the propane tank. Propane tanks are designed to handle much higher than normal operating LPG pressures but it is still important to not exceed the propane tank max pressure rating limit.
The typical propane tank would probably only burst with propane tank max pressure rating over 6895 kPa or 1,000 PSIG. That means the propane tank max pressure rating limit is about 5x the normal operating LPG gas cylinder-bottle pressure.
This would vary by the manufacturer and the cylinder itself.
Propane tanks have LPG pressure relief valves incorporated into the main valve.
The typical LPG pressure relief valve setting is 2585 kPa or 375 PSIG.
So, the propane tank max pressure would never actually go above this, as the valve would open and lets some gas escape, limiting the LPG pressure inside the tank and never even approaching the propane tank max pressure rating limit.
Propane tank pressure varies with temperature.
Even at 70ºC (158ºF), well beyond normal ambient temperatures, the propane tank pressure would only be 2482 kPa (360 PSIG) and still well below the propane tank max pressure rating limit.
So, not only wouldn’t it approach propane tank max pressure rating or bursting pressure limit (≈ 1,000 PSIG) but, under normal circumstances, it would never even reach the 375 PSIG required to trigger the LPG pressure relief valve.
LPG-Propane Pressure Regulator – LPG Working Pressure in Appliances
An LPG-propane pressure regulator is used to reduce and maintain a safe operating LPG gas pressure. Natural gas and LPG-propane appliances use different pressure regulators to operate at different pressures, with 1.1 kPa and 2.75 kPa, respectively.
The actual LPG working pressure in LPG gas appliances is much less than the LPG gas cylinder pressure (LPG gas bottle pressure), at 2.75 kPa.
LPG Gas Cylinder Pressure (LPG gas bottle pressure) – LPG Pressure Temperature Chart
PG gas cylinder pressure (LPG gas bottle pressure) exists when LPG is stored in a gas bottle, as LPG is under pressure. LPG gas cylinder pressure (LPG gas bottle pressure) can vary greatly based on temperature.
The level of fill in the gas bottle comes into play when the LPG is being used, as it affects the rate of LPG vapourisation.
As LPG is a liquefied gas, the LPG gas pressure inside the cylinder will remain the same from full until the last of the liquid LPG is vaporised.
Then the LPG gas cylinder pressure (LPG gas bottle pressure) will fall quickly as the last of the LPG vapour is used, as well.
The use of LPG gas pressure as a volume measurement in LPG use is very limited.
A steady LPG gas cylinder-bottle pressure only shows that liquid LPG is inside the cylinder, but not how much liquid is left.
In the US, LPG vapour pressure is measured in pounds per square inch (PSIG).
PSIG is defined as 1 pound of force applied per square inch as measured by a gauge.
As a comparison, 1 psi = 6.89476 kPa.
What is the LPG Gas Cylinder-Bottle Pressure of a 20 lb Propane Tank? A 9kg LPG Cylinder?
BBQ gas bottles, like a 20lb propane tank or a 9kg gas bottle, have the same LPG gas cylinder-bottle pressure as large gas bottles. LPG gas cylinder-bottle pressure all depends on the temperature.
At 100°F, a 20lb propane tank has 172 PSIG of pressure.
Similarly, a 9kg gas bottle has 1183kPa at 38°C.
This includes, in the UK, Calor gas bottle pressure.
How Much Pressure is in a 100 lb Propane Tankcalor gas bottle pressure
Larger gas bottles, like a 100 lb propane tank or a 45kg gas bottle, have the same LPG gas cylinder-bottle pressure as small gas bottles. LPG gas cylinder-bottle pressure all depends on the temperature.
At 100°F, a 100 lb propane tank has 172 PSIG of pressure.
Similarly, a 45kg gas bottle has 1183kPa at 38°C.
Final Thoughts on LPG Gas Pressure
How much LPG gas cylinder-bottle pressure is dependent upon the temperature of the contents.
This, in turn, is affected by the ambient temperature.
The cylinder is actually capable of handling about 5x the normal LPG gas pressure.
That normal LPG gas cylinder pressure (LPG gas bottle pressure) is far more than what is required for the LPG working pressure of gas appliances.
To reduce the LPG gas pressure, a gas regulator is used between the LPG cylinder and the LPG appliances.
“Safe and Simple” is the best summary of how it all works.
